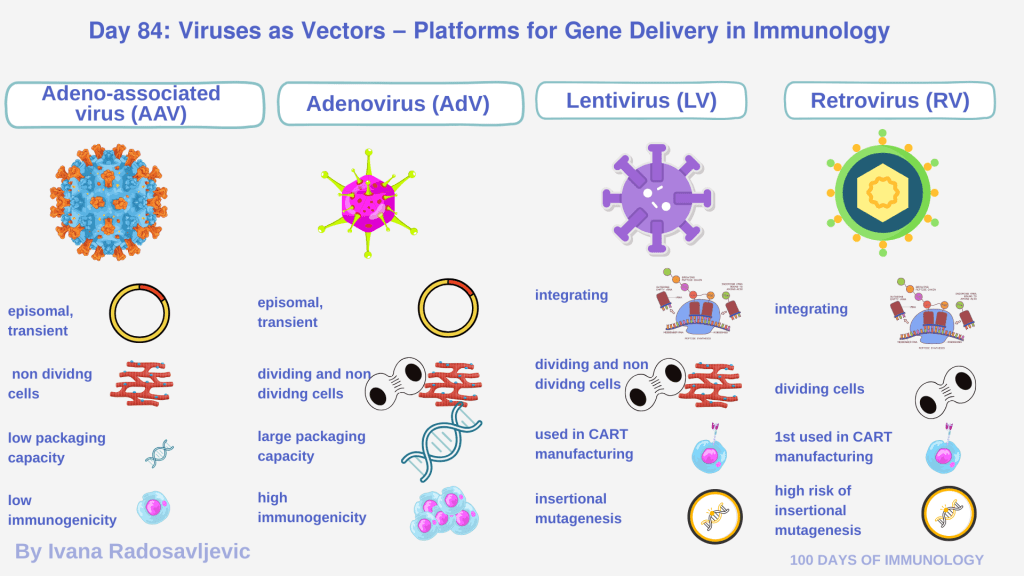
Viruses for gene delivery into cells are Adeno-Associated Virus (AAV), Adenovirus (AdV), Lentivirus (LV), and Retrovirus (RV) [1–3].
During my two-month training in AAV production, I learned that construct size, capsid tropism, packaging cell-line, and vector titer are critical. While working with AAV9, I encountered a challenge: my construct was near the upper size limit for AAV packaging. It was transformative experience for me to realize that what many people fear, viruses, can be harnessed for genetic engineering.
𝗔𝗱𝗲𝗻𝗼𝘃𝗶𝗿𝘂𝘀
• Integration: Episomal (non-integrating) [1, 4]
• Target cells: Dividing and non-dividing
• Pros: Large payload capacity (≈ 30–36 kb), high transduction efficiency, rapid onset of expression [4]
• Cons: High immunogenicity, transient expression, immune elimination [1, 5]
𝗔𝗱𝗲𝗻𝗼-𝗔𝘀𝘀𝗼𝗰𝗶𝗮𝘁𝗲𝗱 𝗩𝗶𝗿𝘂𝘀
• Integration: Episomal in most cases
• Target cells: Non-dividing or slowly dividing cells (muscle, liver, CNS) [2]
• Pros: Low immunogenicity, favorable safety profile, stable long-term expression in many tissues, broad tropism, high vector titers [2]
• Cons: Limited packaging capacity (~4.7–5 kb), restricts delivery of large transgenes; relatively slow onset of transgene expression (1–2 weeks) [2]
𝗟𝗲𝗻𝘁𝗶𝘃𝗶𝗿uses
• Integration: Stable insertion ensures durable transgene expression [3]
• Target cells: Both dividing and non-dividing cells
• Pros: Broad tropism; stable long-term expression; used for ex vivo modification of immune cells (e.g., CAR-T, CAR-NK cells) [3]
• Cons: Risk of insertional mutagenesis; biosafety and vector design; limited payload compared with adenovirus [3]
𝗥𝗲𝘁𝗿𝗼𝘃𝗶𝗿𝘂𝘀
• Integration: Stable insertion into host genome [1]
• Target cells: Dividing cells
• Pros: Stable expression; among the first used vectors in gene therapy applications; useful for dividing hematopoietic cells and early CAR-T development [1, 3]
• Cons: risk of insertional mutagenesis; less flexible tropism compared to LV or AAV [1]
𝗩𝗲𝗰𝘁𝗼𝗿 𝗳𝗼𝗿 𝗜𝗺𝗺𝘂𝗻𝗼𝘁𝗵𝗲𝗿𝗮𝗽𝘆 & 𝗚𝗲𝗻𝗲 𝗘𝗱𝗶𝘁𝗶𝗻𝗴
CAR-T/CAR-NK manufacturing: uses integrating vectors (LV or RV) to ensure stable transgene expression and durable persistence of engineered immune cells in vivo. LV most widely used platform [3].
𝗤𝘂𝗲𝘀𝘁𝗶𝗼𝗻
Which viral vector would you choose for a next-generation CAR-T therapy (+ CRISPR editing) – AAV, LV, RV, AdV?
Stay tuned for 𝗗𝗮𝘆 𝟴𝟱: Shuffling TCRs and Antibodies
𝗥𝗲𝗳𝗲𝗿𝗲𝗻𝗰𝗲𝘀
1. doi: 10.1038/nrg3742
2. DOI: 10.3390/v15030698
3. DOI: 10.2478/raon-2022-0002
4. DOI: 10.1038/nrg1066
5. DOI: 10.3390/cimb45060307
#ViralVectors #GeneTherapy #CAR_T #CRISPRDelivery #AAV #Lentivirus #Retrovirus #Adenovirus #Immunology #TranslationalMedicine #CellTherapy #100DaysOfImmunology