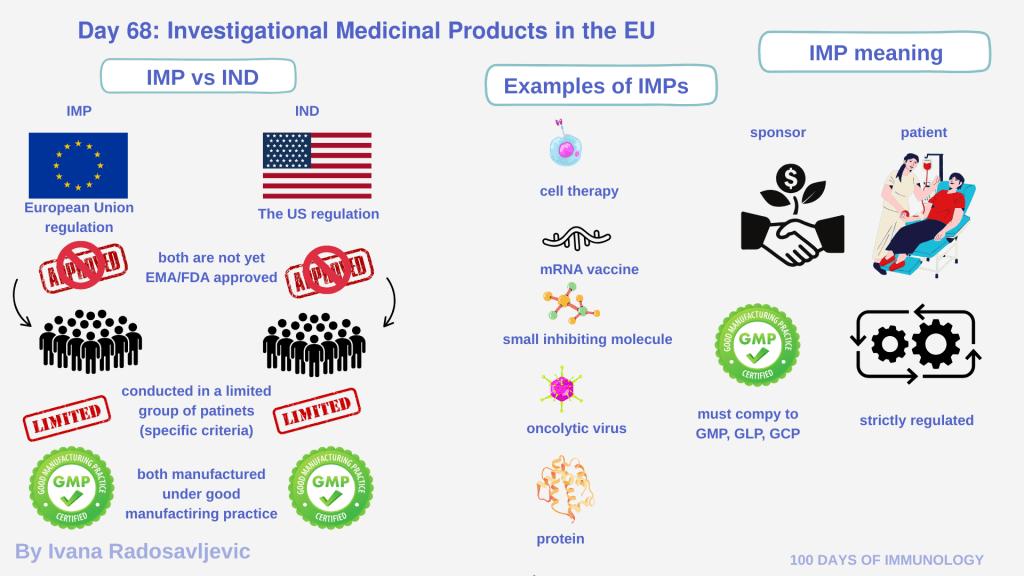
Investigational Medicinal Products (𝘐𝘔𝘗𝘴) are a regulatory classification unique to the European Union, rigorous approach to clinical research and patient safety [1,2]. Unlike in the U.S., where early-stage therapies are covered with Investigationl New Drug (IND)regulations, the EU specifically defines IMPs as medicinal products used in a clinical trial to investigate their safety or efficacy. Therefore; any drug/therapy that is not approved yet is called IMP. That does not mean US does not have its equivalent, they are under different name. The existence of IMP only in the EU does not mean fewer candidates in the US.
Drug developers usually prepare both an IND (US) and an IMP (EU) if they plan to run studies in both regions.
An IMP can be: small molecules, recombinant proteins, gene therapies, or cellular products such as CAR-T cells [3]. For clinical trial sponsors, Contract (Development and) Manufacturing Organizations (C(D)MOs), and Contract Research Organizations (CROs), an IMP is manufactured under strict GMP conditions, just as any approved drug. However, for the patient, receiving an IMP means – participation in a controlled study where the therapy is administered with the purpose of treating disease, not for routine care [4].
These therapies often stem from bench discoveries that are translated into clinical use to exploit newly understood mechanisms.
𝗔𝗻𝗲𝗰𝗱𝗼𝘁𝗲:
I initially struggled to understand how a therapy could be considered an IMP while being administered consecutively in trials. For example, switchable modular CAR-T platforms involves sequential administrations of two components (the CAR-T cell and Targeting Module), both classified as IMPs, each designed to engage immune responses safely and effectively [5].
𝗤𝘂𝗲𝘀𝘁𝗶𝗼𝗻 𝗳𝗼𝗿 𝘁𝗵𝗲 𝗮𝘂𝗱𝗶𝗲𝗻𝗰𝗲: If a CAR-T cell therapy is still an Investigational Medicinal Product (IMP) in the EU, is it less likely to reach patients compared to an EMA-approved CAR-T?
What factors limit access during the IMP phase, and how do approval and reimbursement change that?
Stay tuned for Day 69: 𝗜𝗻𝗱𝗶𝘃𝗶𝗱𝘂𝗲𝗹𝗹𝗲𝗿 𝗛𝗲𝗶𝗹𝘃𝗲𝗿𝘀𝘂𝗰𝗵 𝗶𝗻 𝗚𝗲r𝗺𝗮𝗻𝘆: When Innovation meets unmet Clinical Need
𝗥𝗲𝗳𝗲𝗿𝗲𝗻𝗰𝗲𝘀:
1. https://lnkd.in/e6BSs926
2. https://lnkd.in/eSKhy4WM
3. https://lnkd.in/eXv9tNwX
4. DOI: 10.1200/JCO.2017.73.4186
5. DOI: 10.1080/2162402X.2020.1743036
hashtag#Immunotherapy hashtag#ClinicalTrials hashtag#CAR_T hashtag#IMPs hashtag#EURegulations hashtag#CellTherapy hashtag#GeneTherapy hashtag#TranslationalResearch hashtag#GMP hashtag#CDMO hashtag#CRO