If you live outside Germany, you have probably never heard of these unique clinical pathways — the Individueller Heilvesruch (IHV), Germany’s individual therapeutic attempt. This mechanism fills a critical gap that traditional clinical trials and investor-driven pipelines often ignore.
𝗪𝗵𝘆 𝗗𝗼 𝗪𝗲 𝗡𝗲𝗲𝗱 𝘁𝗵𝗲 𝗜𝗻𝗱𝗶𝘃𝗶𝗱𝘂𝗲𝗹𝗹𝗲𝗿 𝗛𝗲𝗶𝗹𝘃𝗲𝗿𝘀𝘂𝗰𝗵?
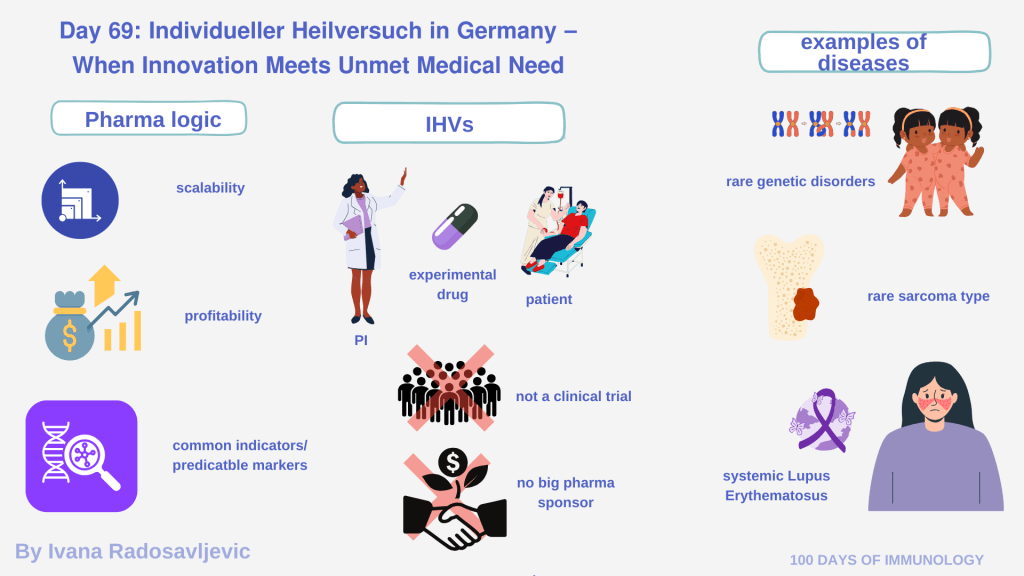
Most pharma development follows the logic of:
• scalability
• profitability
• common indications
• predictable markets
Meaning that rare autoimmune disorders, unusual tumors, and ultra-rare genetic diseases are not profitable enough for investors or industry to pursue them [1].
𝗧𝗵𝗶𝘀 𝗶s 𝘄𝗵𝗲𝗿𝗲 𝗜𝗛𝗩 𝘀𝘁𝗲𝗽s i𝗻:
It enables a physician (PI) to use an experimental, non-approved therapy for a single patient with no remaining standard-of-care options.
• It is 𝘯𝘰𝘵 a clinical trial.
• It is 𝘯𝘰𝘵 sponsored by a pharma company.
• It is not funded by insurance.
The physician or the institution carries the cost and responsibility ethically, medically, and financially [2].
𝗘𝘅𝗮𝗺𝗽𝗹𝗲𝘀
Ultra-rare genetic mutations (e.g., N-of-1 gene therapies)
Aggressive, untreatable solid tumors (e.g., rare sarcoma subtypes)
Autoimmune disorders, such as systemic lupus erythematosus (SLE) [3]
Anecdote
I found out about Individueller Heilversuch by accident. Curious as always, I bombarded my colleague senior scientist with endless questions. He finally directed me to our clinical trial consultant, an expert who once mentioned IHVs in a lecture.
That day, I imagined myself someday as a PI pioneering a new CAR-T, given under an IHV framework to a small group of patients — perhaps for systemic lupus erythematosus or a rare solid tumor subtype.
𝗕𝗲𝗰𝗮𝘂𝘀e 𝗜 𝗯𝗲𝗹𝗶𝗲𝘃𝗲: Every human life matters, even if only one patient in the world has that disease.
𝗜𝗛𝗩𝘀 𝗔𝗿𝗲 𝗘𝘁𝗵𝗶𝗰𝗮𝗹𝗹𝘆 Powerful
They give patients hope where industry pipelines offer none.
They allow scientists and clinicians to test mechanistic discoveries in real humans – years before such an idea could become a commercial product.
They support highly personalized approach.
𝗤𝘂𝗲𝘀𝘁𝗶𝗼𝗻 𝗳𝗼𝗿 𝘁𝗵𝗲 𝗔𝘂𝗱𝗶𝗲𝗻𝗰𝗲: Do you think more countries should adopt the concept?
Stay tuned for 𝗗𝗮𝘆 𝟳𝟬: 𝗥𝗲𝗰𝗮𝗽 𝗼𝗳 𝗜𝗺𝗺𝘂𝗻𝗼-𝗼𝗻𝗰𝗼𝗹𝗼𝗴𝘆 𝘀𝗲𝗿𝗶𝗲𝘀
𝗥𝗲𝗳𝗲𝗿𝗲𝗻𝗰𝗲𝘀
1. DOI: 10.1007/978-90-481-9485-8_27
2. https://lnkd.in/eceReQMJ
3. DOI: 10.1056/NEJMoa2308917
hashtag#Immunology hashtag#ClinicalResearch hashtag#Germany hashtag#IndividuellerHeilversuch hashtag#RareDiseases hashtag#EthicsInMedicine hashtag#CAR_T hashtag#Autoimmunity hashtag#TranslationalMedicine hashtag#100DaysOfImmunology