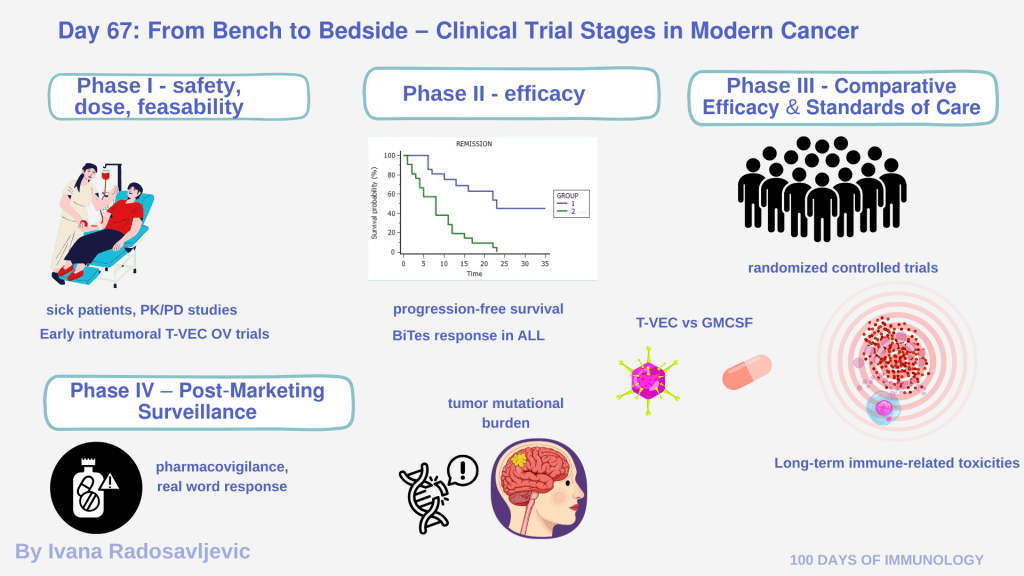
Developing immunotherapies requires navigating a rigorous clinical trial pipeline:
𝗣𝗵𝗮𝘀𝗲 𝗜 – 𝗦𝗮𝗳𝗲𝘁𝘆, 𝗗𝗼𝘀𝗲, 𝗙𝗲𝗮𝘀𝗶𝗯𝗶𝗹𝗶𝘁𝘆
Phase I trials primarily assess safety, dose escalation, and pharmacokinetics/pharmacodynamics (PK/PD) [1].
In classical drug trials, healthy individuals are often recruited.
But CAR-T trials are different: they do not use healthy volunteers because CAR-T cells are living therapies. Instead, they enroll patients with advanced malignancies where potential benefit outweighs immunologic risk [2].
𝗘𝘅𝗮𝗺𝗽𝗹𝗲𝘀:
First-in-human CD19 CAR-T studies leading to Kymriah approval [2]
Early intratumoral T-VEC OV trials [4]
First PD-1 blockade dose-escalation cohorts [3]
𝗔 𝗽𝗲𝗿𝘀𝗼𝗻𝗮𝗹 𝗮𝗻𝗲𝗰𝗱𝗼𝘁𝗲: I once thought CAR-T trials had ´one fewer phase´ and received regulatory approval faster. CAR-T must complete the same four-phase pathway, though adapted for cellular immunotherapies.
𝗣𝗵𝗮𝘀𝗲 𝗜𝗜 – 𝗘𝗳𝗳𝗶𝗰𝗮𝗰𝘆 𝗦𝗶𝗴𝗻𝗮𝗹𝘀
Phase II trials evaluate whether the therapy shows clinical activity in its target population [1]. They assess:
• Objective Response Rate (ORR)
• Duration of Response (DoR)
• Progression-Free Survival (PFS)
𝗘𝘅𝗮𝗺𝗽𝗹𝗲:
BiTEs showing responses in relapsed ALL [5]
Phase III – Comparative Efficacy and Standards of Care
Phase III consists of large randomized controlled trials (RCTs) comparing new therapies to standard treatments [1]. They assess:
• Overall Survival (OS)
• Quality of Life (QoL)
• Long-term immune-related toxicities
𝗘𝘅𝗮𝗺𝗽𝗹𝗲𝘀:
• T-VEC vs GM-CSF pivotal melanoma study [4]
• Pembrolizumab dominating multiple tumor types in RCTs [3]
𝗣𝗵𝗮𝘀𝗲 𝗜𝗩 – 𝗣𝗼𝘀𝘁-𝗠𝗮𝗿𝗸𝗲𝘁𝗶𝗻𝗴 𝗦𝘂𝗿𝘃𝗲𝗶𝗹𝗹𝗮𝗻𝗰𝗲
Examines:
• Real-world effectiveness
• Rare or delayed toxicities (e.g. long-term CRS)
• Outcomes in broader demographics
• Pharmacovigilance programs [1]
𝗛𝗼𝘄 𝗪𝗲 𝗠𝗲𝗮𝘀𝘂𝗿𝗲 𝗧𝗿𝗶𝗮𝗹 𝗦𝘂𝗰𝗰𝗲𝘀𝘀
Effectiveness is assessed using:
• OS, PFS, EFS
• Tumor mutational burden (TMB)
• Immune infiltration (CD8+, Tregs)
• Biomarkers (PD-L1, CD19, BCMA, EGFRvIII)
𝗤𝘂𝗲𝘀𝘁𝗶𝗼𝗻 𝗳𝗼𝗿 𝘁𝗵𝗲 𝗔𝘂𝗱𝗶𝗲𝗻𝗰𝗲
Which immunotherapy modality do you think faces the highest barrier in progressing from Phase II to Phase III?
Stay tuned for 𝗗𝗮𝘆 𝟲𝟴: 𝗜𝗻𝘃𝗲𝘀𝘁𝗶𝗴𝗮𝘁𝗶𝗼𝗻𝗮𝗹 𝗠𝗲𝗱𝗶𝗰𝗶𝗻𝗮𝗹 𝗣𝗿𝗼𝗱𝘂𝗰𝘁𝘀 𝗶𝗻 𝘁𝗵𝗲 𝗘𝗨
𝗥𝗲𝗳𝗲𝗿𝗲𝗻𝗰𝗲𝘀
1. DOI: 10.1016/j.canlet.2025.217616
2. DOI: 10.1126/science.aar6711
3. DOI: 10.1126/science.aar4060
4. DOI: 10.1038/s41573-019-0029-0
5. DOI: 10.3390/cancers15184550
hashtag#Immunology hashtag#Oncology hashtag#ClinicalTrials hashtag#CancerResearch hashtag#CAR_T hashtag#BiTEs hashtag#OncolyticViruses hashtag#CheckpointInhibitors hashtag#TranslationalResearch hashtag#Immunotherapy hashtag#RegulatoryScience hashtag#100DaysofImmunology