𝗜𝗺𝗽𝗼𝗿𝘁𝗮𝗻𝗰𝗲 𝗼𝗳 𝗣𝗼𝘀𝗶𝘁𝗶𝘃𝗲 𝗮𝗻𝗱 𝗡𝗲𝗴𝗮𝘁𝗶𝘃𝗲 𝗖𝗼𝗻𝘁𝗿𝗼𝗹𝘀
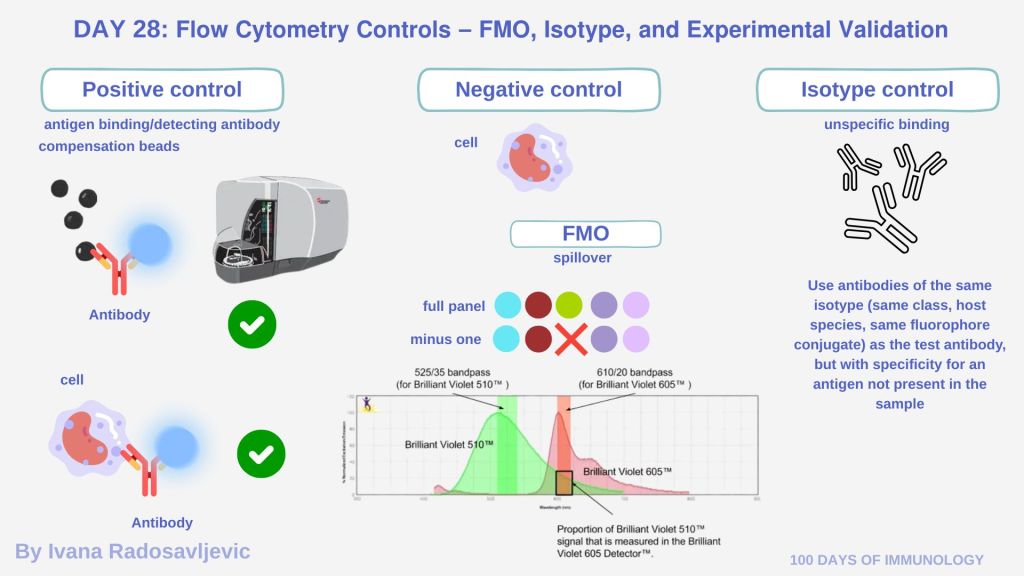
𝘗𝘰𝘴𝘪𝘵𝘪𝘷𝘦 𝘤𝘰𝘯𝘵𝘳𝘰𝘭𝘴 are samples that express the antigen of interest or samples stained with each individual antibody separately. They ensure that each antibody binds correctly to its epitope and that the cytometer detects the signal. In many flow cytometry setups (e.g., Navios), positive controls mean 𝘤𝘰𝘮𝘱𝘦𝘯𝘴𝘢𝘵𝘪𝘰𝘯 𝘣𝘦𝘢𝘥𝘴. Bead-based positive controls are useful for instrument calibration, they are not 100% representative of biological signal.
𝘕𝘦𝘨𝘢𝘵𝘪𝘷𝘦 𝘤𝘰𝘯𝘵𝘳𝘰𝘭𝘴 include unstained samples (cells but with no antibody) to assess background fluorescence and autofluorescence. Negative controls help set gates and thresholds.
𝗪𝗵𝗮𝘁 𝗔𝗿𝗲 𝗙𝗠𝗢, 𝗜𝘀𝗼𝘁𝘆𝗽𝗲, 𝗮𝗻𝗱 𝗘𝘅𝗽𝗲𝗿𝗶𝗺𝗲𝗻𝘁𝗮𝗹 𝗩𝗮𝗹𝗶𝗱𝗮𝘁𝗶𝗼𝗻 𝗖𝗼𝗻𝘁𝗿𝗼𝗹𝘀
𝘍𝘔𝘖 (𝘍𝘭𝘶𝘰𝘳𝘦𝘴𝘤𝘦𝘯𝘤𝘦 𝘔𝘪𝘯𝘶𝘴 𝘖𝘯𝘦) 𝘊𝘰𝘯𝘵𝘳𝘰𝘭𝘴: samples stained with all fluorophores in a panel except one. It helps determine how much 𝘴𝘱𝘪𝘭𝘭𝘰𝘷𝘦𝘳 from other channels affects the channel in which the omitted fluorophore would be detected. It is especially 𝘶𝘴𝘦𝘧𝘶𝘭 𝘸𝘩𝘦𝘯 𝘮𝘢𝘳𝘬𝘦𝘳 𝘦𝘹𝘱𝘳𝘦𝘴𝘴𝘪𝘰𝘯 𝘪𝘴 𝘭𝘰𝘸 𝘰𝘳 𝘵𝘩𝘦 𝘱𝘰𝘱𝘶𝘭𝘢𝘵𝘪𝘰𝘯 𝘪𝘴 𝘤𝘰𝘯𝘵𝘪𝘯𝘶𝘰𝘶𝘴 rather than having a clear positive/negative separation [1].
𝘐𝘴𝘰𝘵𝘺𝘱𝘦 𝘊𝘰𝘯𝘵𝘳𝘰𝘭𝘴: Use antibodies of the same isotype (same class, host species, same fluorophore conjugate) as the test antibody, but with specificity for an antigen not present in the sample. They help assess nonspecific binding due to the antibody backbone or Fc receptor binding. [2].
𝘌𝘹𝘱𝘦𝘳𝘪𝘮𝘦𝘯𝘵𝘢𝘭 𝘝𝘢𝘭𝘪𝘥𝘢𝘵𝘪𝘰𝘯 𝘊𝘰𝘯𝘵𝘳𝘰𝘭𝘴: These may include known positive and negative cell lines or primary cells, stimulated vs unstimulated samples, or other treatment controls to show that your staining protocols, fixation, and flow cytometer settings are working as intended.
𝗟𝗮𝗯 𝗧𝗶𝗽𝘀 𝗳𝗼𝗿 𝗨𝘀𝗶𝗻𝗴 𝗖𝗼𝗻𝘁𝗿𝗼𝗹𝘀
Always include single-stain controls for every fluorophore in your panel to set compensation [1]. When marker expression is low (e.g. CD14’s HLA-DR^lo/neg subset) [3], FMO controls are critical to avoid false positives.
𝗤𝘂𝗲𝘀𝘁𝗶𝗼𝗻 Which control(s) do you find indispensable in your flow cytometry experiments – and why?
Stay tuned for 𝗗𝗮𝘆 𝟮𝟵: 𝗩𝗶𝘀𝘂𝗮𝗹𝗶𝘇𝗶𝗻𝗴 𝗗𝗮𝘁𝗮 – 𝗗𝗼𝘁 𝗣𝗹𝗼𝘁𝘀, 𝗛𝗶𝘀𝘁𝗼𝗴𝗿𝗮𝗺𝘀, 𝗮𝗻𝗱 𝗚𝗮𝘁𝗶𝗻𝗴 𝗔𝗽𝗽𝗿𝗼𝗮𝗰𝗵𝗲𝘀
𝗥𝗲𝗳𝗲𝗿𝗲𝗻𝗰𝗲𝘀
1. https://www.bio-rad-antibodies.com/flow-cytometry-fmo-controls.html
2. https://docs.abcam.com/pdf/secondary-antibodies/multicolor-flow-cytometry-guide.pdf
3. doi: 10.18632/aging.202571
#100DaysOfImmunology #FlowCytometry #Controls #FMO #IsotypeControl #ExperimentalValidation #ImmunologyMethods #SingleCellTechniques