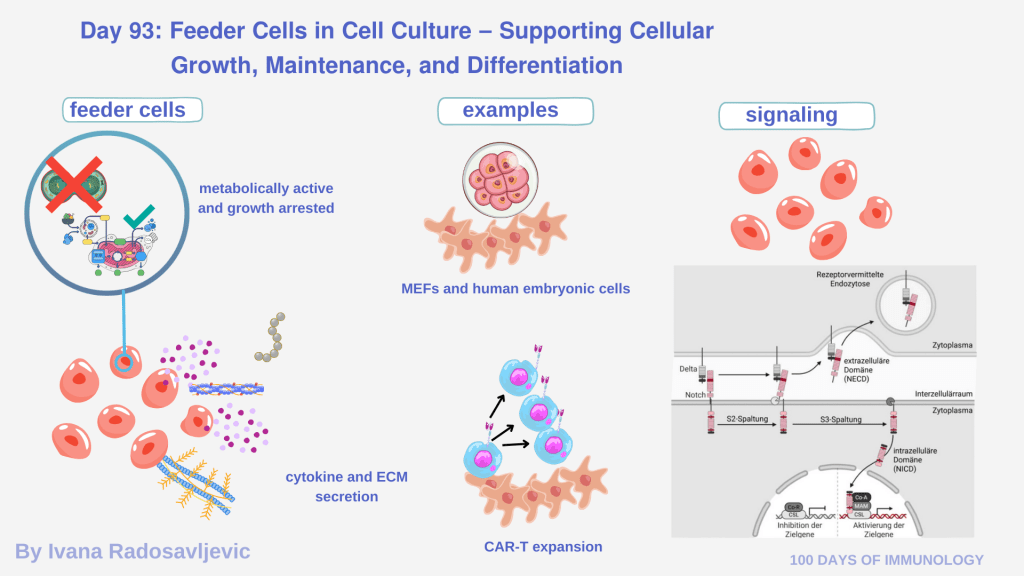
Feeder cells are growth-arrested yet metabolically active cells used to support the survival, proliferation, and differentiation of more fragile/ demanding target cells. Feeder cells are feeding main cells: feeder layers actively shape the cellular microenvironment by secreting growth factors, cytokines, extracellular matrix (ECM) components, and metabolic support signals that cannot be fully replaced by media supplements alone [1].
𝗖𝗹𝗮𝘀𝘀𝗶𝗰𝗮𝗹 𝗲𝘅𝗮𝗺𝗽𝗹𝗲𝘀 include irradiated or mitomycin C–treated mouse embryonic fibroblasts (MEFs) for human embryonic stem cells and induced pluripotent stem cells, as well as stromal feeder cells supporting hematopoietic progenitors and lymphocytes [2]. In immunology, feeder cells are critical in co-culture systems, such as T cell activation assays, CAR-T expansion protocols, and thymic selection models, where cell–cell contact, membrane-bound ligands, and localized cytokine gradients determine functional outcomes [3].
Feeder cells enable 𝗲x𝗽𝗲𝗿𝗶𝗺𝗲𝗻𝘁𝗮𝗹 𝗰𝗼𝗻𝘁𝗿𝗼𝗹 𝗼𝘃𝗲𝗿 𝘀𝗶𝗴𝗻a𝗹𝘀 like Notch ligands, IL-7, IL-15, or costimulatory molecules, allowing researchers to decouple intrinsic immune cell programming from environmental cues. Their use introduces complexity, batch variability, and translational challenges, which is why feeder-free and artificial antigen-presenting systems are also explored in clinical manufacturing [4].
Question for the Audience: Are feeder cells irreplaceable part of research or a technical limitation we should urgently engineer away?
Stay tuned for 𝗗𝗮𝘆 𝟵𝟰: 𝗙𝗲𝗲𝗱𝗲𝗿-𝗜𝗻𝗱𝗲𝗽𝗲𝗻𝗱𝗲𝗻𝘁 𝗖𝗲𝗹𝗹 𝗘𝘅𝗽𝗮𝗻𝘀𝗶𝗼𝗻 – 𝗔𝗱𝘃𝗮𝗻𝗰𝗶𝗻𝗴 𝗦𝗰𝗮𝗹𝗮𝗯𝗹𝗲 𝗮𝗻𝗱 𝗖𝗼𝗻𝘁𝗿𝗼𝗹𝗹𝗲𝗱 cell 𝗖𝘂𝗹𝘁𝘂r𝗲 𝗦y𝘀𝘁𝗲𝗺𝘀
𝗥𝗲𝗳𝗲𝗿𝗲𝗻𝗰𝗲𝘀
1. DOI:10.1002/9780470649367
2. DOI: 10.1126/science.282.5391.1145
3. DOI: 10.3389/fimmu.2024.1453740
4. DOI: 10.1016/j.ymthe.2017.03.007
#CellCulture #FeederCells #Immunology #CoCulture #StemCells #CAR_T #TranslationalResearch #100DaysOfImmunology