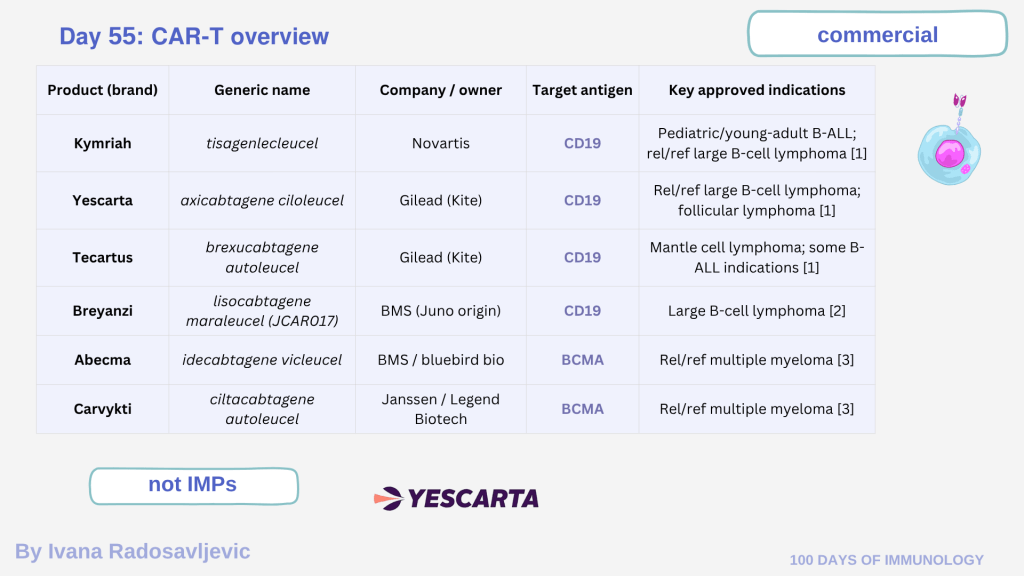
Seven CAR-T products have received FDA approval for hematologic malignancies (targeting CD19 or BCMA). An overview ⬇️
Product (brand) I Generic name I Company / owner I Target antigen I Key approved indications
Kymriah I tisagenlecleucel I Novartis CD19 Pediatric/young-adult B-ALL; rel/ref large B-cell lymphoma [1]
Yescarta I axicabtagene ciloleucel I Gilead (Kite) I CD19 I Rel/ref large B-cell lymphoma; follicular lymphoma [1]
Tecartus I brexucabtagene autoleucel I Gilead (Kite) I CD19 I Mantle cell lymphoma; some B-ALL indications [1]
Breyanzi I lisocabtagene maraleucel (JCAR017) I BMS (Juno origin) I CD19 Large B-cell lymphoma [2]
Abecma I idecabtagene vicleucel I BMS / bluebird bio I BCMA I Rel/ref multiple myeloma [3]
Carvykti I ciltacabtagene autoleucel I Janssen / Legend Biotech I BCMA Rel/ref multiple myeloma [3]
𝗨𝗻𝗱𝗲𝗿𝘀𝘁𝗮𝗻𝗱𝗶𝗻𝗴 𝘁𝗵𝗲 𝗰𝗼𝗻𝘁𝗲𝘅𝘁
𝘛𝘢𝘳𝘨𝘦𝘵𝘴: CD19-CAR-Ts for B-cell cancer treatment; BCMA-CAR-Ts target plasma cells in myeloma.
𝘙𝘦𝘨𝘶𝘭𝘢𝘵𝘰𝘳𝘺 𝘴𝘵𝘢𝘵𝘶𝘴: All six products passed FDA review and are available as commercial therapies (not IMPs). Clinical-stage CAR-Ts, by contrast, remain IMPs (Investigational Medicinal Products) [1][3].
𝘊𝘰𝘮𝘮𝘦𝘳𝘤𝘪𝘢𝘭 𝘷𝘴 𝘤𝘭𝘪𝘯𝘪𝘤𝘢𝘭: Commercial = marketed, licensed, and reimbursed. Clinical/experimental = used only in trials under IND or CTA.
𝘙𝘦𝘮𝘪𝘴𝘴𝘪𝘰𝘯 & 𝘳𝘦𝘭𝘢𝘱𝘴𝘦: CAR-T often induces complete remission (CR) in refractory patients, yet relapse may occur due to antigen escape or T-cell exhaustion [1][2].
𝗡𝗮𝗺𝗶𝗻𝗴
Generic name – Axicabtagene ciloleucel = an engineered (gene) T-cell (leucel) product targeting CD19 via CAR technology.
𝗪𝗵𝘆 𝘀𝗼 𝗲𝘅𝗽𝗲𝗻𝘀𝗶𝘃𝗲?
CAR-T therapy is a living, patient-specific biologic drug, it involves:
Autologous leukapheresis → manufacturing → multi-week expansion → QC testing
Transport across continents, manufacturing under GMP ´clean room´ conditions
Inpatient monitoring for CRS/neurotoxicity
Regulatory burden [1][5].
𝗤𝘂𝗲𝘀𝘁𝗶𝗼𝗻: Which challenge do you think matters most for the next generation of CAR-T?
Stay tuned for 𝗗𝗮𝘆 𝟱𝟲: 𝗖𝗔𝗥-𝗧 𝘀𝗶𝗱𝗲 𝗲𝗳𝗳𝗲𝗰𝘁𝘀
𝗥𝗲𝗳𝗲𝗿𝗲𝗻𝗰𝗲𝘀
1. DOI: 10.46989/001c.124277
2. https://www.bms.com/assets/bms/ca/documents/productmonograph/BREYANZI_EN_PM.pdf
3. DOI: 10.3389/fimmu.2022.991092
4. https://www.reuters.com/business/healthcare-pharmaceuticals/us-fda-eliminates-risk-evaluation-mitigation-strategies-car-t-cancer-therapies-2025-06-27/
5. DOI:10.18609/cgti.2020.058
#CAR_TCells #Immunotherapy #Hematology #CellTherapy #CancerResearch #BiotechInnovation #PrecisionMedicine #TranslationalResearch #FDAApproved #OncologyCommunity #BioManufacturing #ImmunoOncology #AcademicScience #BiomedicalEngineering #TCellTherapy