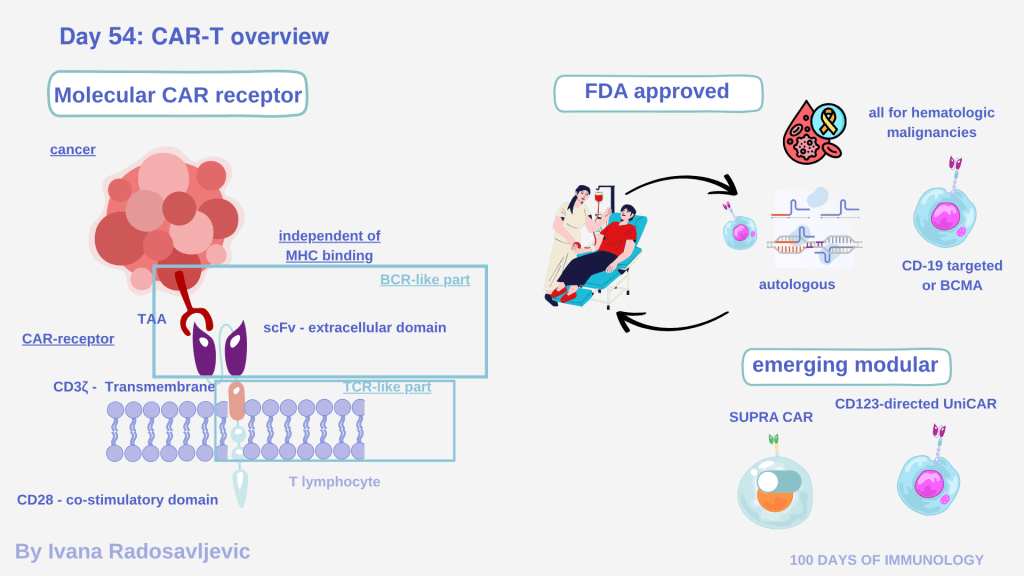
𝘊𝘩𝘪𝘮𝘦𝘳𝘪𝘤 𝘈𝘯𝘵𝘪𝘨𝘦𝘯 𝘙𝘦𝘤𝘦𝘱𝘵𝘰𝘳 𝘛-𝘤𝘦𝘭𝘭 (𝘊𝘈𝘙-𝘛) therapy represents one of the most transformative breakthroughs in cancer immunotherapy. Mechanistically, CAR-T cells are genetically engineered T lymphocytes that express a chimeric receptor – so called because it fuses structural elements of both B-cell receptors (BCRs) and T-cell receptors (TCRs) into one synthetic molecule [1].
The extracellular single-chain variable fragment (scFv) recognizes a tumor antigen independently of MHC, mimicking the antigen-binding domain of BCRs, while the intracellular tail combines TCR-derived signaling (CD3ζ) with one or more co-stimulatory domains (CD28, 4-1BB) – together ensuring robust activation, proliferation, and persistence [2][3].
𝗔𝘂𝘁𝗼𝗹𝗼𝗴𝗼𝘂𝘀 𝘃𝘀. 𝗔𝗹𝗹𝗼𝗴𝗲𝗻𝗲𝗶𝗰 As explained in previous post [4].
𝗖𝘂𝗿𝗿𝗲𝗻𝘁 𝗟𝗮𝗻𝗱𝘀𝗰𝗮𝗽𝗲
To date, six CAR-T products have received FDA approval—including Breyanzi®, Kymriah®, Yescarta®, Carvykti®, Tecartus® and Abecma®— primarily for hematologic malignancies like B-cell acute lymphoblastic leukemia and multiple myeloma [5]. Solid tumors remain a challenge due to the immunosuppressive tumor microenvironment (TME), poor trafficking, and antigen heterogeneity [6].
𝗠𝗼𝗱𝘂𝗹𝗮𝗿 𝗮𝗻𝗱 𝗡𝗲𝘅𝘁-𝗚𝗲𝗻𝗲𝗿𝗮𝘁𝗶𝗼𝗻 𝗖𝗔𝗥𝘀 ‘
Emerging 𝘮𝘰𝘥𝘶𝘭𝘢𝘳 𝘊𝘈𝘙𝘴 such as UniCAR, SUPRA-CAR, and OmniCAR introduce ´on-off´ and ´switchable´ architectures, allowing tunable targeting and safety control through adaptor molecules [7]. CAR-T evolution went through three generations – each adding co-stimulatory layers – and now the fourth-generation ´armored CARs´ and logic-gated synthetic designs with built-in cytokine secretion or checkpoint inhibition [8].
𝗦𝗽𝗲𝗰𝘂𝗹𝗮𝘁𝗶𝘃𝗲 𝗵𝘆𝗽𝗼𝘁𝗵𝗲𝘀𝗶𝘀: Why are CAR-Ts so popular despite their long and costly clinical pipeline? They embody the ideal of personalized, living drugs – a therapy that literally evolves with the patient. Even with complex logistics, the promise of reprogramming one’s immune system to recognize and destroy cancer remains irresistibly powerful.
Stay tuned for 𝗗𝗮𝘆 𝟱𝟱: 𝗙𝗗𝗔 𝗮𝗽𝗽𝗿𝗼𝘃𝗲𝗱 𝗖𝗔𝗥-𝗧𝘀
𝗥𝗲𝗳𝗲𝗿𝗲𝗻𝗰𝗲𝘀:
1. DOI: 10.1056/NEJMra1706169
2. DOI: 10.1007/978-3-540-73259-4_14
3. DOI: 10.1158/2159-8290.CD-12-0548
4. DOI: 10.1038/s41573-019-0051-2
5. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products
6. DOI: 10.1038/s41408-021-00459-7
7. DOI: 10.1016/j.imlet.2019.05.003
8. DOI: 10.1038/s41416-018-0325-1
#100DaysOfImmunology #CARTCells #CellTherapy #ImmunoOncology #SyntheticBiology #ModularCAR #AdoptiveCellTherapy #CancerImmunology #HematologicMalignancies #SolidTumors